Best Practices for Patient Discharge Planning & Medication Management
When it comes to Patient Discharge Planning and Medication Management, there is a lot to consider in order to collect, consolidate and communicate patient discharge information in an effective and timely manner. Throughout this blog, we’ll explore the best practices for medication management, including the Irish Pharmacy Union’s IPU Product File as well as a solution for creating and communicating electronic patient discharge summaries.
The HIQA National Standard for Patient Discharge Summary Information
A National Standard for Patient Discharge Summary Information was published in 2013. It provides guidance to hospitals on the information they need to include in a patient discharge summary.
It delivers benefits for the hospital as it improves the efficiency of the patient discharge process. There are benefits for G.P.’s and Primary Care Practitioners as it provides a standardised patient discharge summary and gives improved and more consistent information on that discharge summary. This results in more effective transfers of patients from the hospital to the community.
There are benefits to the patient as the patient discharge summary facilitates the transfer of relevant clinical information between clinicians and it improves the consistency and the quality of that information; thereby improving overall patient safety.
The National Standard for Patient Discharge Summary Information Covers 7 Groups
- Patient Details
- Primary Care Healthcare Professional Details
- Admission and Discharge Information
- Clinical Information
- Medication Information
- Follow up and Future Management
- Person completing Discharge Summary
The Irish Pharmacy Union
The Irish Pharmacy Union (IPU) is the professional representative organisation for community pharmacy in Ireland. It has over 2200 members representing 95% of community pharmacies in the country. Services provided by the IPU include professional advice and guidance, business support, continuous professional development and support with Pharmaceutical Society of Ireland and HSE primary care reimbursement service issues. Another support the IPU provides to pharmacist members and to other organisations by way of license is their Medicinal Product Catalogue- the IPU Product File.

The IPU Product File and Virtual Medicinal Management in eDischarge
The National Health Products Catalogue was launched in an effort to help progress the implementation of a National Medicinal Products catalogue. This has long been a key deliverable of the ePharmacy program within eHealth Ireland. The key aim of their medicinal catalogue is to provide a consistent approach to the identification and naming of medicines.
Recently VMP’s have been added to the IPU Product File. A VMP is an abstract representation of an active substance associated with strength information and route of administration. A VMP describes the generic title for a product including form and strength- examples being Atenolol 100 mg tablets or Atorvastatin 40 mg tablets.
The background to this is that in 2003 ePrescribing was identified in the National eHealth strategy as a key priority for Ireland. HIQA subsequently defined the data model required to support the implementation of an electronic medicinal product reference catalogue or in other words an electronic dictionary of medicines that could be used for prescribing and dispensing.
Below is the data model that had been referenced by HIQA to support the development of a national electronic Medicinal products catalogue and it consists of three virtual concepts and three actual concepts as shown on this slide.
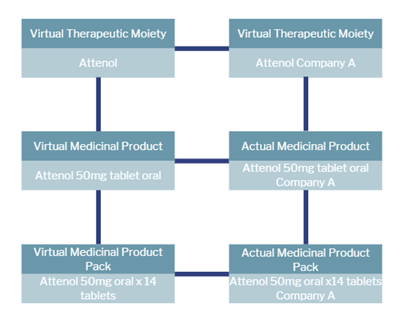
The virtual concepts are shown on the left-hand side above and they refer to pharmacological information. They are independent of supplier or jurisdiction and they identify pharmaceutical concepts that may be used in the actual medicinal products. The actual concepts are displayed on the right-hand side of the model and are used to identify actual medicinal products that are produced by manufacturers and are available in the Irish market for prescribing and dispensing.
The IPU undertook a project on their Medicinal products catalogue to add VMP information to the file so that it would be in line with the HIQA data model and therefore could be used to support Ireland’s ePharmacy initiatives.
The project has been a success and has resulted in a large number of actual medicinal products on the IPU Product File being linked to a VMP. There are currently hospitals using the IPU’s VMP information to for ePrescription and eDischarge. The benefits of these systems are many; they include reduced medication errors, reduced Adverse Drug Events (ADE’s), improved communication between healthcare workers and the secure printing and transmission of prescriptions.
The National Release Centre for SNOMED in Ireland
The VMP project which the IPU worked on fed nicely into another project they have also been working on which is the National Release Centre (NRC) for SNOMED in Ireland mapping project. In 2014 HIQA recommended the adoption of SNOMED CT as the clinical terminology for Ireland and in 2016 Ireland became the 29th member of SNOMED international. This is exciting for Healthcare information standards and data quality in Ireland as it represents a huge step forward to be using an internationally recognised, robust classification standard that can support eHealth initiatives. The objective of SNOMED-CT is to facilitate the accurate recording and sharing of clinical healthcare information.

One of the benefits realised from the adoption of SNOMED-CT is the reduction of boundary effects which typically arise where different terminologies or coding is used by different clinicians and departments, and between different organisations and indeed countries.
SNOMED-CT enables clinical information to be recorded using consistent, common terminology and supports the sharing of information between healthcare providers.
To further enhance the IPU Product File, the IPU have started mapping concepts within our file including our VMP’s to the clinical drugs within SNOMED-CT. While their initial matching success rate for the VMPS has been quite low with a matching success rate of 40% this project is ongoing.
Electronic Patient Discharge with GeneCIS eDischarge
GeneCIS eDischarge facilitates Patient Discharge planning and Medications management. Key information on Diagnosis, Chief complaints, Procedures and Treatments, Clinical Findings and Tests, Infection control, known allergies, Medication and Progress and Treatment on discharge are all recorded within the solution and on the discharge summary letter once validated. This means that complete and accurate records of care are produced at discharge. The Discharge Summary is accessible instantly to all authenticated users of the solution. As the solution is simple to use and intuitive it can be deployed with a minimum amount of user training. Patient safety is most important and with GeneCIS eDischarge the handover of care between the Hospital clinician and the G.P is better managed and safer. If the patient is readmitted, their previous admission history is available and instantly accessible so there are efficiency gains also to be realised from adoption of the solution.

_______________________________________________________________________________
Watch the On-Demand Webinar
Best Practices for Patient Discharge Planning and Medication Management which includes a demonstration of the IPU Product File in use in GeneCIS eDischarge.
_______________________________________________________________________________
Medication Management with eDischarge
Within the Medications panel, eDischarge automatically connects to the VMP once it has been integrated. To add a new medication a user just needs to select ‘Add New Record’. While this is selected the system will continue to display a list of medications previously saved so that the user is continually reminded of what medications have already been added for the patient.
When searching for a medication to add the user can search by the Trade name or by the Generic name. Once selected the user is prompted to enter the dose, the frequency, the route of administration, the duration and if there are any comments these can also be recorded here. They can also tick whether the patient was admitted to hospital on that drug or not. Once saved this will be added to the list of medications recorded for the patient.
Once all the medications are selected the user can then create the ePrescription from within the GeneCIS eDischarge solution by selecting the drugs they want to place on the prescription. When generating the prescription any medication that is not selected at this stage will be moved to discontinued on the eDischarge summary letter.
GeneCIS ePrescription enhances the Discharge workflow process as the medications on the Discharge summary automatically transfers to the prescription which saves time as it eliminates the need for further data capture. Medication Errors and Adverse Drug Events are also reduced when this need for further transcription is taken out of the equation. It facilitates oversight by hospital pharmacists. For clinicians it provides better communication around modifications made to the patients prescription during the inpatient stay. It is faster too as the ePrescription can be sent electronically by HL7 messaging. For G.P’s and Pharmacists the absence of legibility issues that accompany hand-written prescriptions means that less time is spent on seeking clarification from the Hospital which the patient is discharged from.

How often is the IPU Product File updated and how is it updated within the GeneCIS eDischarge solution?
The VMP file is currently sent out on a monthly basis by the IPU Product file team to the hospitals that are using it. In the future the IPU are planning a web-based system from which users can download and then update the IPU Product File more often, even on a daily basis if they so wish.
Within GeneCIS eDischarge, a system administrator can take the new file and automatically import it into the system on a monthly basis which is a controlled process. DMF Systems also offer training on how to do this.
How do you manage brand specific drugs within the VMP file?
All medicines that are actual medicines that fit under a particular VMP will be listed under the VMP. Once you search within the VMP name all the actual medicines will show up under that drop down. All medicines whether they have a trade name or not will show up under those results.
What if an error has been made on a GeneCIS eDischarge report and is only noticed after it is printed: How does the system or user cope with this scenario?
GeneCIS eDischarge supports the ability to clone a Discharge summary in the event that an error has been made on a patient discharge summary. Where the error is noticed the user can clone the existing letter and add a note detailing that the patient discharge summary has been updated. The user would then simply update the patient discharge summary, save it and it will automatically be sent to the G.P as an updated patient discharge letter.
What’s the process for medication management if the medicine is not listed?
GeneCIS ePrescription supports the addition of free text in situations where the medicine is not listed, so it can be entered as free-text and it will appear on the prescription as a free text box.
How do you manage drugs that won’t be on the IPU file until the next update?
The IPU Product File is kept as up to date as possible. As soon as a new drug comes on to the Market it will be added to the IPU Product File and while it is currently updated on a monthly basis, it will soon be possible to download an updated Product File every day. It is rare that something would be added to the market before the IPU have had the opportunity to update the file. Drug manufacturers would know to contact the IPU when they are adding a new drug to the market. The file is very up to date.
How does the GeneCIS eDischarge solution allow custom or ad hoc reports to be created?
Using Microsoft Power BI, we can connect to the GeneCIS eDischarge database to support the creation of custom of ad hoc reports. There is a training course around that which can be incorporated as part of the implementation of GeneCIS eDischarge. Out-of-the-box, GeneCIS eDischarge comes with a number of generic reports around utilisation, but additional reports can be created using Microsoft Power BI.
Does the GeneCIS eDischarge software support readmission?
If a patient is readmitted and they have lots of episodes of care then the GeneCIS eDischarge summary application will allow you to create a discharge summary against all or any one of those episodes of care as you require. For patient safety reasons we do not support cloning of patient discharge summaries across episodes of care so users would be required to create a new discharge summary for that readmission.
I hope the above has provided you with some insights on how technology such as GeneCIS eDischarge and ePrescriptions can support medication management and enhance communication between primary and secondary care. If you have any question or would like to learn more information, don’t hesitate to get in touch at info@dmf.ie and we’ll be happy to help!









